What is an Atom ?
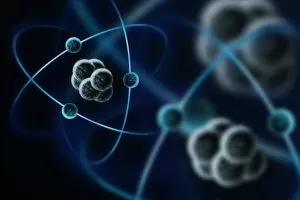
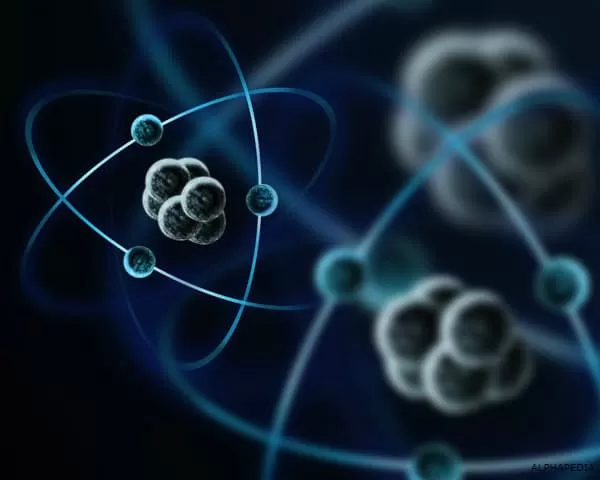
The atom is the smallest object that retains the properties of an element. They are the so-called basic units of matter. They structure all the elements in a definitive way. The word “atom” has its origin from the Greek meaning indivisible, because it was once believed that atoms were the smallest things in the universe and could not be divided.
We now know that atoms are made up of three particles: protons, neutrons, and electrons. And that they’re also made up of even smaller particles, like quarks. Atoms were created after the Big Bang more than 13.7 billion years ago. When the universe heated up, became more dense, and then cooled down, the right conditions were created for the formation of quarks and electrons. Quarks joined together to form protons and neutrons, and these particles combined into nuclei. All this took place within the first minutes of the universe’s existence, according to CERN.
It took about 380,000 years for the universe to cool down, which caused the electrons to slow down, so the nuclei could capture them to form the first atoms. These were primarily hydrogen and helium, which remain the most abundant elements in the universe, according to Jefferson Lab. Gravity eventually caused the gas clouds to coalesce and form stars, and heavier atoms were (and still are) created within the stars and sent out into the universe when the star exploded (supernova).
Atomic Particles
Protons and neutrons are heavier than electrons and reside in the nucleus at the center of the atom. The electrons are very light and are in a kind of cloud that orbits the nucleus of the atom. The cloud of electrons extends to a radius 10,000 times greater than the nucleus. Protons and neutrons have about the same mass. However, a proton is about 1,835 times more massive than an electron.
Atoms always have the same number of protons and electrons, and the number of protons and neutrons is also usually the same. Adding a proton to an atom creates a new element, while adding a neutron creates an isotope, or a heavier version, of that atom. The chemical behavior of an atom is determined by the arrangement of its electrons. Atoms combine with each other through chemical bonds to produce so-called molecules. They can also ionize and form so-called ionic compounds.
The Protons
Protons are positively charged particles within the nuclei of atoms. Protons are approximately 99.86% as massive as neutrons. The number of protons in an atom is unique to each element, and is known as the atomic number. The number of protons also determines the chemical behavior of the element.
The Electrons
Electrons are small compared to protons and neutrons, more than 1800 times smaller. The electrons are negatively charged and are electrically attracted to the positively charged protons. The electrons surround the atomic nucleus in paths called orbitals.
The Neutrons
Neutrons are uncharged particles found inside all atomic nuclei (except hydrogen). The mass of a neutron is slightly larger than that of a proton.
Related Topics

ENVIRONMENT DEFINITION

DEFINITION OF ART

INOCULATING LOOP DEFINITION

💚 WHAT IS ENGINEERING

DEFINITION OF ARGUMENT

DEFINITION OF SOUL
Other Topics of Interest in ALPHAPEDIA

DEUTERONOMY 31:8

FREE MASTER DEGREE IN OCCUPATIONAL HEALTH

FREE WORDPRESS COURSE

FREE BACHELOR DEGREE IN INDUSTRIAL DESIGN

FREE CISCO COURSE

FREE BACHELOR DEGREE IN ORTHOPEDIC
Atom Definition Image